Stroke remains a devastating complication of sickle cell anemia (SCA). The STOP (Stroke Prevention Trial in Sickle Cell Anemia) protocol adopted in 2014 by the National Heart Lung and Blood Institute (NHLBI) guidelines includes stroke-risk screening using transcranial Doppler ultrasound (TCD) and prevention with chronic red cell transfusion therapy (CRCT). Theserecommendations were reinforced by the American Society of Hematology guidelines.However, despite the evidence supporting TCD screening in stroke prevention, the DISPLACE study (Dissemination and Implementation of Stroke Prevention Looking at the Care Environment) showed that real-world implementation was insufficient.
To evaluate and improve sickle stroke screening, we conducted the NHLBI-funded multi-center DISPLACE study to identify barriers to TCD implementation and test novel methods for improving outcomes. DISPLACE is a 4-part study: 1) Retrospective calculation of TCD screening rates; 2) Assessment of barriers and facilitators; 3) Implementation trial to improve TCD screening and 4) Comparison of implementation sites to controls. Importantly, DISPLACE is the largest prospective study of children with SCA. This study employed a multi-level, multi-faceted cluster-randomized study of interventions to improve implementation rates of TCD.
Methods: DISPLACE is a 28-center US consortium representing a range of: regions (urban vs rural), size (patient number), and institution type (academic vs community). In Part 1, sites performed a rigorous retrospective review of TCD screening from 2012-2016 to collect baseline rates. Part 2 included a barriers and facilitators assessment to TCD screening to inform the trial interventions: 1) Re-branding TCD as Sickle Stroke Screen, a meaningful name for SCD stakeholders; 2) Using a Single Coordinator (SC) for each site's TCD scheduling, and follow-up; 3) A custom designed provider-focused application, ProviderMinder TM (PM) created to remind providers to track TCD appointments and follow-up on results. When a TCD was due, PM alerted the provider to enter scan results or reason(s) missed. Once the TCD results (normal/conditional/abnormal) were entered in PM, providers were prompted to enter the next TCD date and/or intervention plan (if not normal). If a result was not entered, PM alerted the provider q3 days until the TCD was recorded as completed or rescheduled.
The implementation trial (parts 3 and 4 of DISPLACE) included 16 sites that had the lowest TCD rates in part 1. The implementation trial included children with SCA aged 2-7 years (the group at highest risk for an abnormal TCD). Cluster randomization was used to allocate sites to PM alone (8 sites) or PM + SC (8 sites).
The primary outcome was comparison of TCD rates between PM and PM+SC sites. Secondary outcomes included comparisons between 1) Part 3 sites' TCD rates and Part 1 baseline rates 2) Part 3 sites and control sites (to assess the impact of the educational rebranding of TCD).
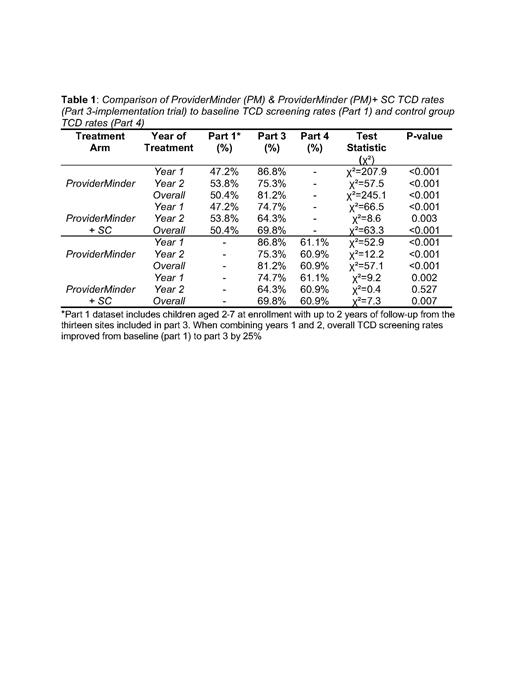
Results: Part 1 included 28 centers with 5246 children with SCA. Of the 16 intervention sites, 3 sites could not implement the interventions due to administrative issues but remained in the study and were re-designated as “control sites” and a 4th control site was added (they were not informed prospectively to avoid bias). Thus, there were 13 evaluable sites for the primary endpoint including 776 patients; 484 in sites using PM and 292 in sites using PM+SC. Control sites included 185 patients. The trial demonstrated superiority of the PM arm compared to the PM + SC arm (P<.0001 year 1 and p=0.002 year 2) in both years of implementation and in comparison, to the control sites (P<.0001 year 1, p=.009 year 2) with an overall improvement in mean TCD screening from part 1 by 25% (table 1).
Conclusions: This was a highly successful implementation study in SCA. This study emphasizes the importance of performing barrier and facilitator assessments when introducing new guideline-recommended screenings into clinical practice. These findings also highlight the importance of ongoing quality assurance in SCA to ensure prevention practices are maintained. Despite the benefit of TCD (sickle stroke screening) in children with SCA, implementation was poor prior to these interventions. Results from this study will guide ongoing optimization of TCD screening and demonstrate the importance of adopting these interventions in the real world.
Disclosures
Kanter:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Austin Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Bluebird Bio: Consultancy; Guidepoint Global: Consultancy; Beam: Consultancy, Honoraria; Vertex: Consultancy, Honoraria; Bausch: Honoraria; Watkins, Lourie, Roll&Chance: Consultancy; Chiesi: Honoraria, Membership on an entity's Board of Directors or advisory committees; Fulcrum: Consultancy; ECOR1: Consultancy. Adams:Zeriscope, Inc: Other: Co-founder ; Duke University, Univ of Miami, Global Blood Therapeutics, Pfizer, Novo-Nordisk: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal